The newly developed adhesive is available in two different grades. Loctite WT 3001 and Loctite WT 3003 have both been tested according to ISO 10993 standards, ensuring a high level of safety and performance for users. The solutions have also been tested for skin sensitization, making them suitable for a wide range of medical applications including wearable devices.
With the increasing demand for medical devices and wearables, the need for safe and reliable adhesive solutions has never been more crucial. Henkel's new wearable device light cure adhesive provides an excellent bonding solution for medical devices, ensuring strong and durable connections while remaining free of known skin sensitizing monomers such as Isobornyl Acrylate (IBOA). IBOA is known in that medical community for causing patient skin reactions when used in wearables.
”At Henkel, we are committed to providing our customers with innovative adhesive solutions that meet the highest safety standards,” said Philipp Loosen, Vice President and Head of Industrials EIMEA and Global Key Accounts Medical at Henkel. ”With the introduction of our new medical grade wearable device light cure adhesive, we are addressing the growing need for safe and effective adhesives in the medical industry. Our adhesive is ISO 10993 tested and formulated with patient comfort and safety in mind, ensuring its suitability for use in medical device bonding and medical wearables.”
Henkel's wearable device light cure adhesive offers various benefits for medical applications. Its light cure capabilities allow for quick and efficient bonding, reducing production time and increasing productivity. The adhesive also provides excellent adhesion to a wide range of substrates commonly used in medical devices and wearables, ensuring robust bonding and sealing performance after drop impact or bathing.
Furthermore, the adhesive's formulation meets FDA & EU MDR recommendations for wearable medical devices regarding sensitizing and CMR ingredients. Henkel is committed to meeting regulatory requirements and delivering safe and reliable adhesive solutions to its customers. The focus on removing all known skin sensitizing monomers further reduces the risk of skin sensitization making the adhesive suitable for prolonged skin contact in medical applications.
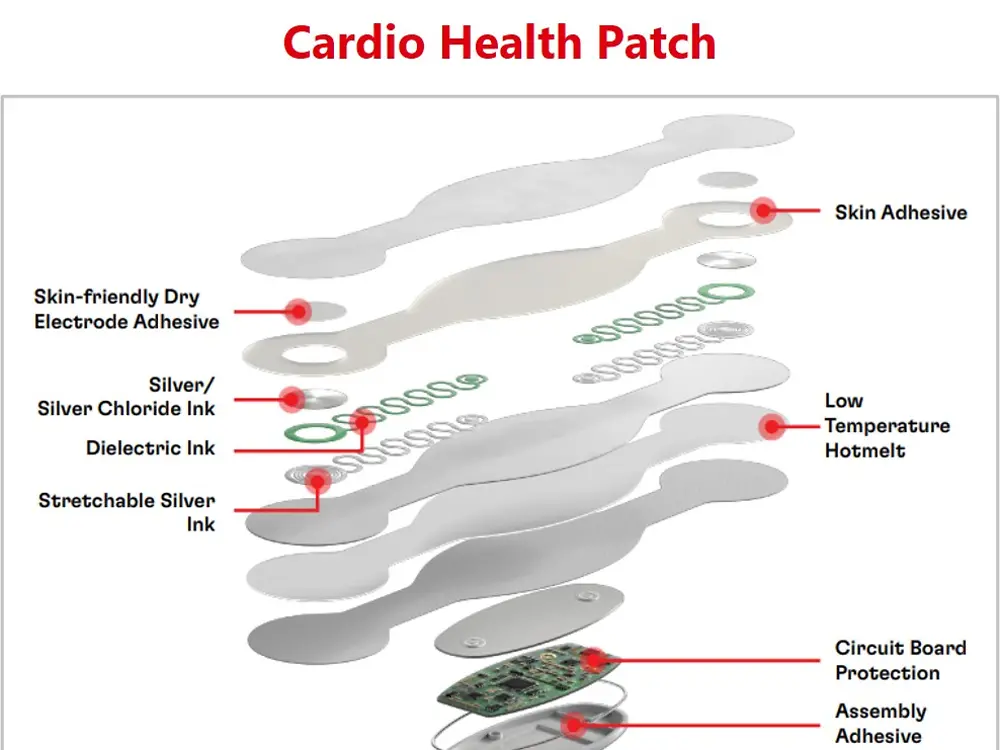
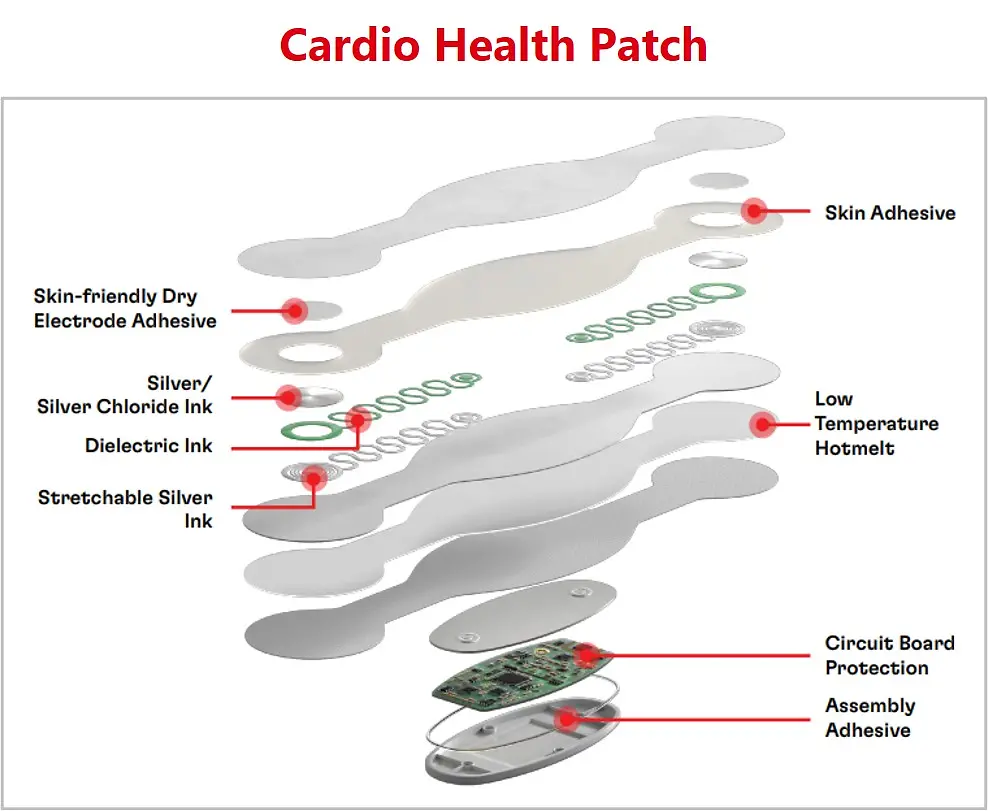
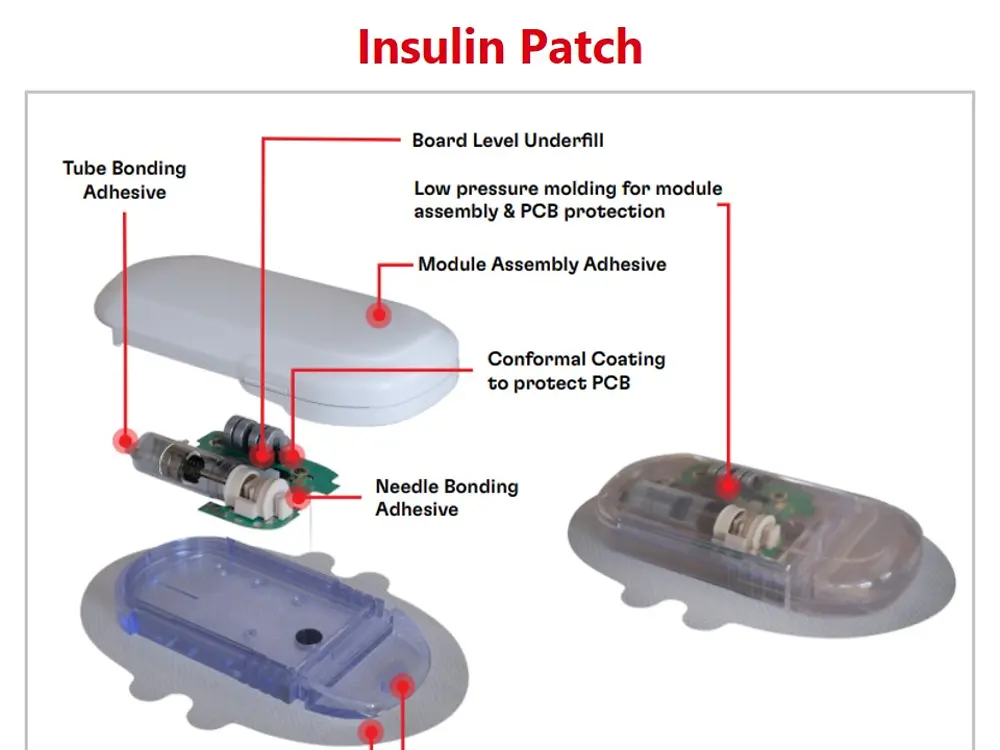
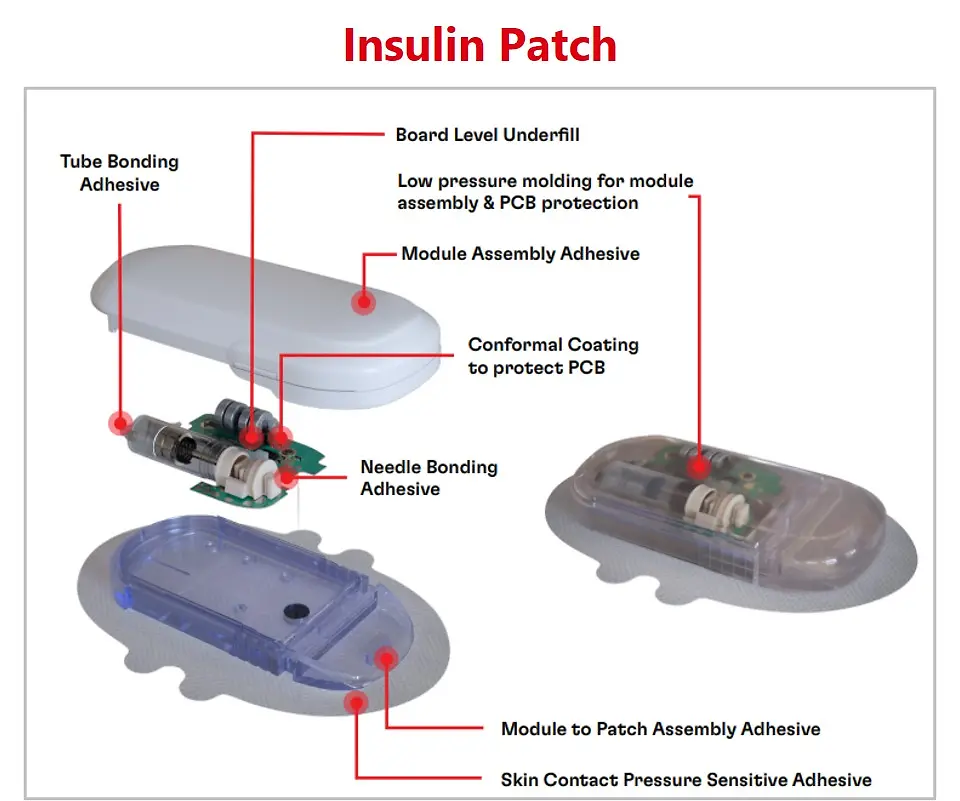
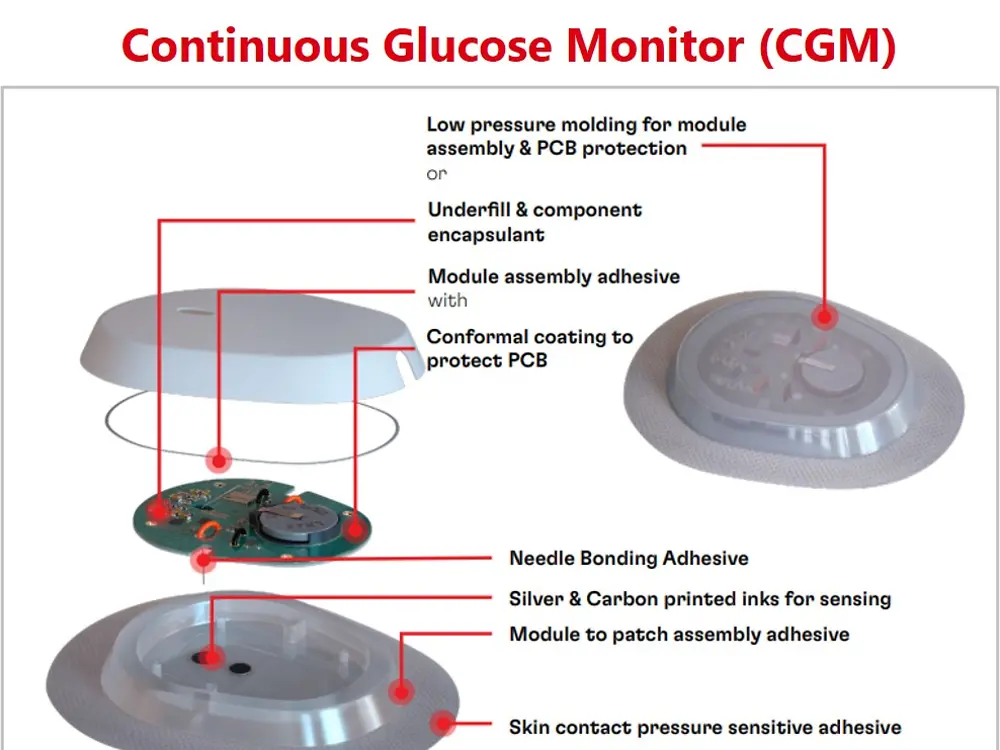
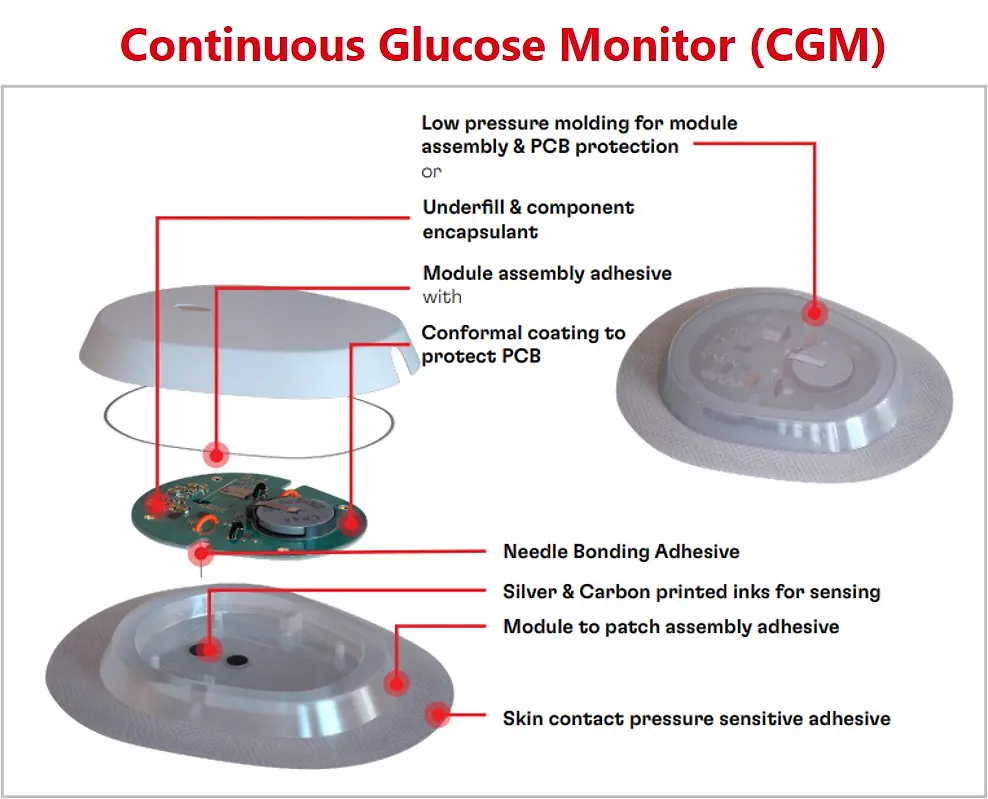
With the trend of the integrated and connected devices, medical wearables look to incorporate advanced technologies and data-driven approaches into the healthcare industry. Henkel offers a broad material solutions portfolio for medical wearables, including low-pressure molding technologies, skin bonding, assembly adhesives, circuit board protection, and printable conductive/functional inks for biosensors.
For more information about Henkel's new medical-grade wearable device light cure adhesive, please visit the Henkel booth at the WT Pavilion at MEDICA (12D33) from November 13 through November 16 in Dusseldorf, Germany or please visit Medical Wearable & Electronic Devices - Henkel Adhesives (henkel-adhesives.com)