Henkel´s and Origin´s first FDA compliant, sterile, 3D printed nasopharyngeal test swab now shipping
With the onset of the COVID-19 pandemic, the demand for medical supplies, including testing kits has outpaced supply. The primary protocol involves conducting a nasopharyngeal (NP) swab test, which collects viral material from inside a person’s nasal cavity. Globally, there are very few companies that manufacture the swabs commercially, and all are overwhelmed with demand. In collaboration with Origin, Henkel is leveraging its ability to carry out biocompatibility and robust mechanical testing at its facility in Concord, California, and has provided a range of 3D printable medical photopolymers designed for use on Origin’s material development systems.
Traditionally, the swabs are made from multiple materials and adjoined prior to sterilization and packaging. Working cooperatively with Origin, Henkel supplied knowledge and technology to help design a swab that could be 3D printed at scale.
“From inception, the vision behind Henkel’s Open Materials Platform was to enable collaboration all along additive manufacturing’s value chain,” says Ken Kisner, Head of Innovation - 3D Printing at Henkel. “Working together with Origin, we were able to develop a product which is very effective as its mass-produced counterparts. With the constraints commercial medical suppliers are facing, this presents a significant opportunity for the 3D printing industry to demonstrate its capabilities, beyond prototyping.”
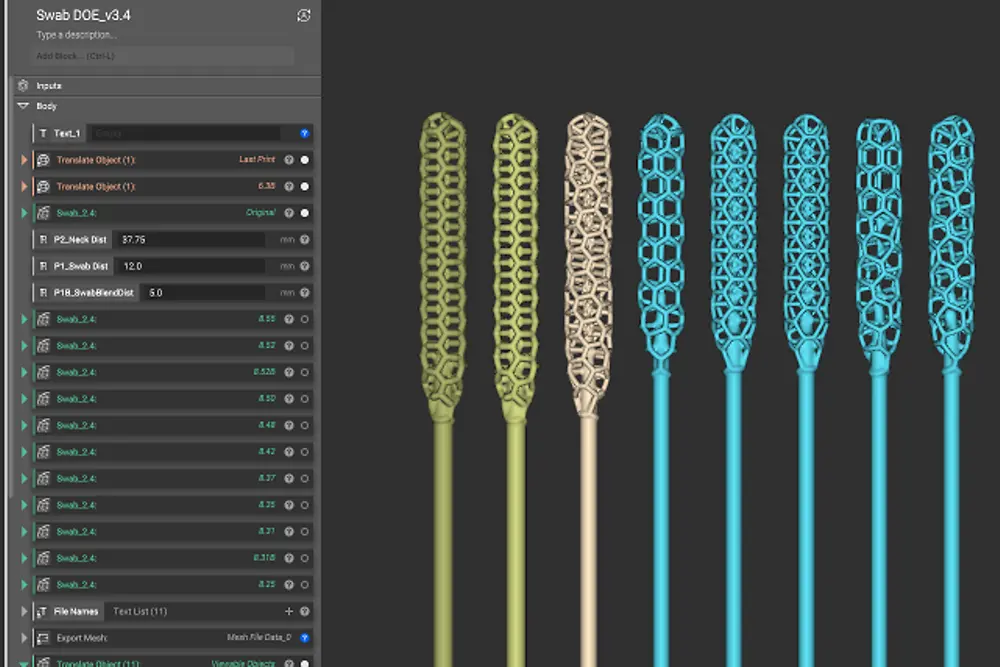
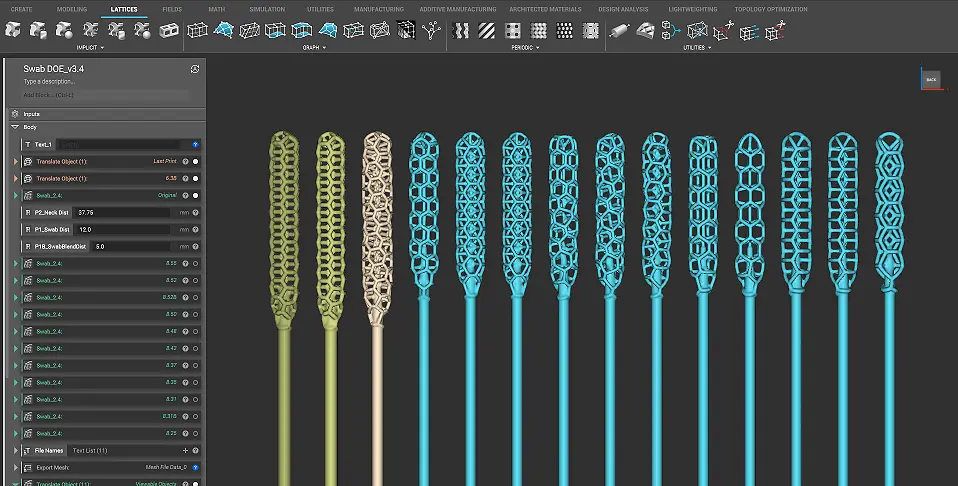
Origin’s programmable photo polymerization (P3) technology enabled the company to test different materials, print parameters, and designs in parallel to find an optimal solution in just a few days. The final clinically validated swab utilizes an intricate lattice design to collect the virus sample. It balances patient comfort with the ability to collect a reliable and sufficient sample.
The development effort also leveraged Henkel’s Albert software platform, which accelerates the innovation of new products by determining the materials that are utilized. With its ability to access Loctite’s vast material and post-processing knowledge and data, Albert allowed Henkel to recommend the best performing material for the NP swab application.
“Nasal swabs are actually very complex instruments,” says Nick Talken, CTO of Albert Software at Henkel. “The head of the swab utilizes a detailed lattice structure that’s designed to maximize the amount of virus collected. The whole thing has to be flexible and strong, not to mention safe for medical use. With the help of Henkel’s Albert Software, we were able to quickly match the best Loctite material for this specific application.”
Leading the effort, Origin also collaborated with Beth Israel Deaconess Medical Center (BIDMC) on the swab’s development. BIDMC conducted a rigorous initial clinical evaluation for human factors, materials testing and ensured that the final product would be compatible with the polymerase chain reaction (PCR) method that is used in laboratories to test for COVID-19.
“By working collaboratively and utilizing each other’s technologies, we identified, optimized and scaled the manufacturing process to bring an application to market extremely fast,” says Chris Prucha, Founder and CEO at Origin.
Origin’s NP swab is classified as a sterile device and is considered a finished medical product. This means it must adhere to “current good manufacturing practices” (cGMP) which are regulations enforced by the FDA and documented in the Code of Federal Regulations (title 21, part 820.) Both Henkel and Origin collaborated to conduct testing and validate each step in the sterilization process, which includes Ethylene Oxide (EtO) and autoclave sterilization. They also performed mechanical testing and intensive packaging validation which determined the product’s FDA compliant shelf life.
Furthermore, the NP swabs are 100% traceable and contain a digital thread that can be traced back to the manufactured base chemistries, which were produced using Albert software. Origin’s sterile NP swabs are currently shipping to leading healthcare facilities, government institutions, and independent testing centers in the U.S. and several other countries.
Discover how the two companies were able to design, develop, validate and scale the NP Swab in this upcoming deep dive webinar with Origin CEO, Chris Prucha and Ken Kisner, Head of Innovation – 3D Printing at Henke on June 26, 2020. Register under: https://us02web.zoom.us/webinar/register/7215917432020/WN_RGk-sFk0R426KBbVYC8ZNw
About Origin
Based in San Francisco, CA, Origin is pioneering the concept of Open Additive Manufacturing, a new way to build based on open materials, extensible software, and modular hardware. Origin One, the company's manufacturing-grade 3D printer, uses programmable photopolymerization to precisely control light, heat, and force among other variables to produce parts with exceptional accuracy and consistency. The company works with a network of material partners to develop a wide range of commercial-grade materials for its system, resulting in some of the toughest and most resilient materials in additive manufacturing. The company was founded in 2015 and is led by alumni from Google and Apple. Investors include Floodgate, DCM, Mandra Capital, Haystack, Stanford University, and Joe Montana. Learn more about Origin here: origin.io